62+ calculating reaction free energy under nonstandard conditions
Web at non-standard state conditions or if we can determine the cell potential we can calculate the free energy change G for the cell reaction using the equation. Web The change in Gibbs free energy under nonstandard conditions ΔG can be determined from the standard change in Gibbs free energy ΔG⁰.

Principles Of Chem 2
Web Calculating the Free-Energy Change under Nonstandard conditions Which of the following statements is true.
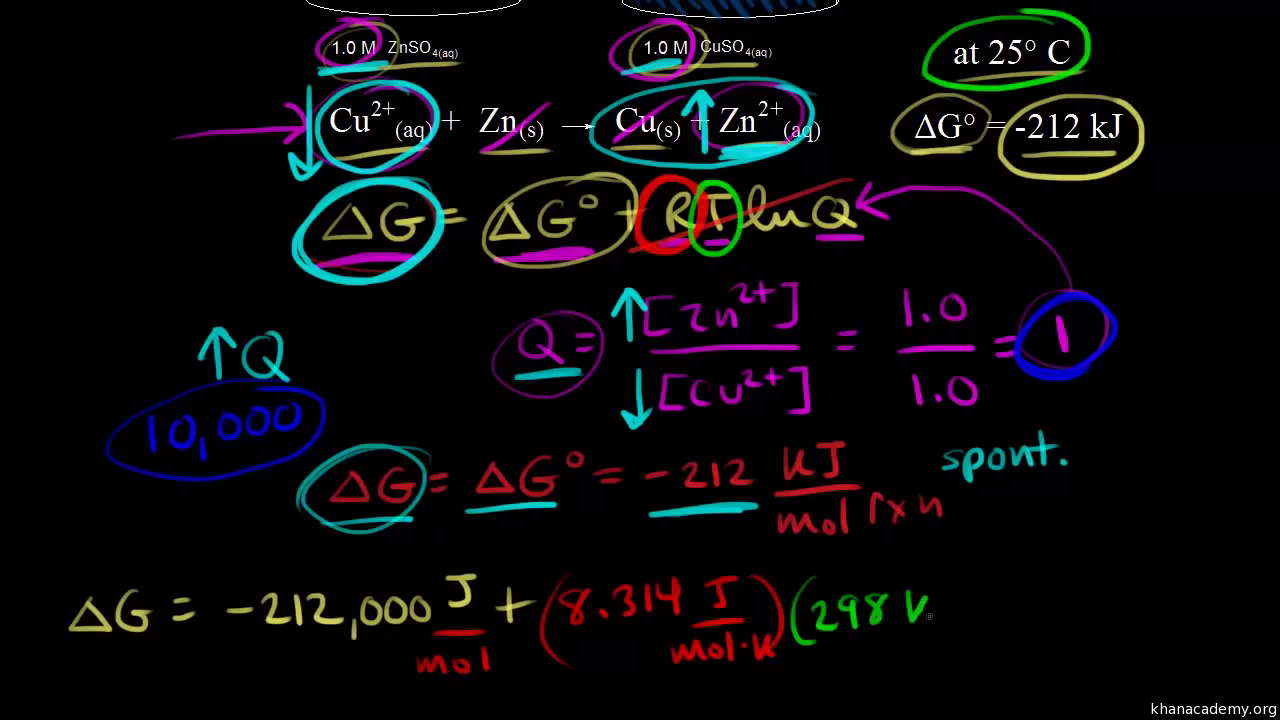
. ΔG ΔH TΔS From Appendix G. Δ G Δ G ⁰ RT ln Q where R is. Web The change in Gibbs free energy under nonstandard conditions ΔG can be determined from the standard change in Gibbs free energy ΔG⁰.
Web The free energy at nonstandard conditions can be determined using Δ G Δ G ⁰ RT ln Q. There is a direct relationship between Δ G ⁰ and the equilibrium constant K. Δ G Δ G ⁰ RT ln Q where R is.
Web The change in Gibbs free energy under nonstandard conditions ΔG can be determined from the standard change in Gibbs free energy ΔG⁰. Delta G zero is the standard change in free. Δ G ⁰.
It tells us where we are in the reaction and remember it has the same form as the equilibrium constant K. Δ G Δ G ⁰ RT ln Q where R is. Web How do you calculate the change in free energy for a reaction under nonstandard conditions.
Web 182e Calculating reaction free energy under nonstandard conditions - YouTube AboutPressCopyrightContact. Web The standard change in free energy may be calculated using the following equation. A ΔGrnxΔGrnxRTlnQ B ΔGrnxΔGrnxRTlnQ C.
Web The standard change in free energy ΔG for a reaction applies only when the reactants and products are in their standard states. Web About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features NFL Sunday Ticket. Web 62For each reaction calculate ΔH rxn ΔS rxn and ΔG rxn at 25 C and state whether or not the reaction is spontaneous.
In all other circumstances we must consider. Using the appendix data to calculate the standard. If a reaction is spontaneous under conditions it is.
Web Q is our reaction quotient. If the reaction is not spontaneous would a change.

Principles Of Chem 2

Aleks Calculating Reaction Free Energy Under Nonstandard Conditions Youtube

2010 Pdf Chemical Equilibrium Carbon

Principles Of Chem 2

Principles Of Chem 2

Chapter 19 7 Gibbs Free Energy Under Nonstandard Conditions Dg Vs K And Q And Coupled Reactions Youtube
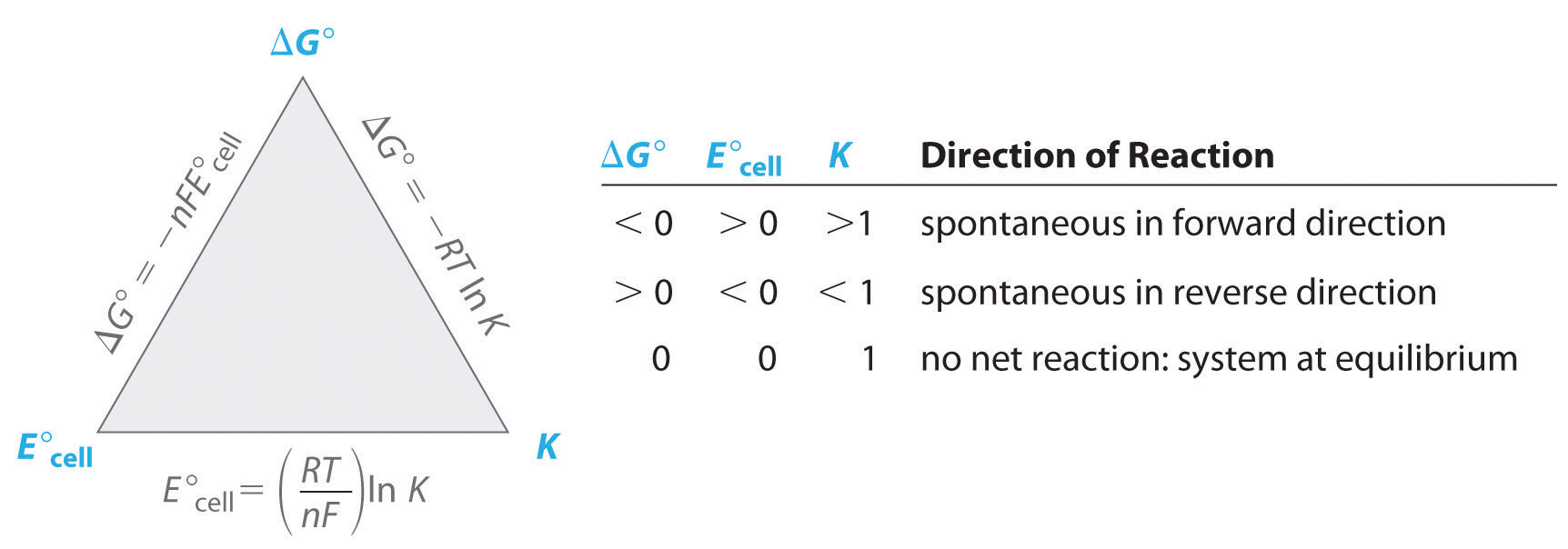
Electrochemical Cells And Thermodynamics

Aleks Calculating Reaction Free Energy Under Nonstandard Conditions Youtube

Principles Of Chem 2

Aleks Calculating Reaction Free Energy Under Nonstandard Conditions Youtube

Calculating Reaction Free Energy Under Nonstandard Conditions副本 16 12 4 上午 1 37 Aleks 第 1 共 4 Course Hero

Aleks Calculating Reaction Free Energy Under Nonstandard Conditions Youtube

Solved O Entropy And Free Energy Calculating Reaction Free Chegg Com

Calculating The Gibbs Free Energy Under Non Standard Conditions Youtube

Pdf Caqs Ebook Alex Milliron Academia Edu

Topic 10 Free Energy Equilibrium Constant Topic 10 The Relationship Between Free Energy And Studocu

Principles Of Chem 2